1.3 Heat and Temperature
In this section, we will explore the concepts of heat and temperature, their definitions, and their significance in thermodynamics.
Temperature: A Measure of Energy State
Temperature is a measure of the internal energy of a substance—specifically, the average energy associated with the microscopic motion of atoms and molecules. In gases, this includes translational, rotational, and vibrational motion. In solids, it's often modeled in terms of lattice vibrations and (in conductors) free electron motion.
From your thermodynamics background, you may recall that temperature correlates with the kinetic energy of particles. However, it's not energy in transit—it’s a state variable that characterizes the thermal condition of a system.
Thermodynamic temperature is the basis for all temperature scales. It is an absolute measure, with zero representing a state of no thermal motion (absolute zero).
Heat: Energy in Transit
Heat, on the other hand, is energy in motion. Specifically, it is energy transferred due to a spatial temperature difference:
\[ \text{Heat transfer} \equiv \text{energy in transit due to } \nabla T \]
Whenever a temperature gradient exists in a medium or between media, heat transfer will occur until thermal equilibrium is reached (Bergman, Lavine, and Incropera, 2019, 2–3).
Definitions, Symbols, and Units
Following Bergman, Lavine, and Incropera, 2019, we make the following definitions and try to maintain consistent notation:
- Temperature: A measure of the average kinetic energy of the molecules in a substance. It is a state variable that determines the direction of heat transfer.
- Symbol: \(T\)
- Units: K (kelvin), °C (degrees Celsius)
- Heat: Energy transferred across the boundary of a system due to a temperature difference. It is not a property of the system but a transient quantity.
- Symbol: \(Q\)
- Units: J (joules)
- Heat transfer rate: The time rate of heat transfer to or from a surface or control volume.
- Symbol: \(\dot{q}\)
- Units: W (watts), J/s (joules per second)
- Heat transfer rate per unit length: The rate of heat transfer per unit length in one-dimensional or line heat flow applications (e.g., fins).
- Symbol: \(q'\)
- Units: W/m
- Heat flux: Heat transfer rate per unit area, often used to characterize surface heating or cooling.
- Symbol: \(q''\)
- Units: W/m²
Modes of Heat Transfer
There are three primary mechanisms of heat transfer:
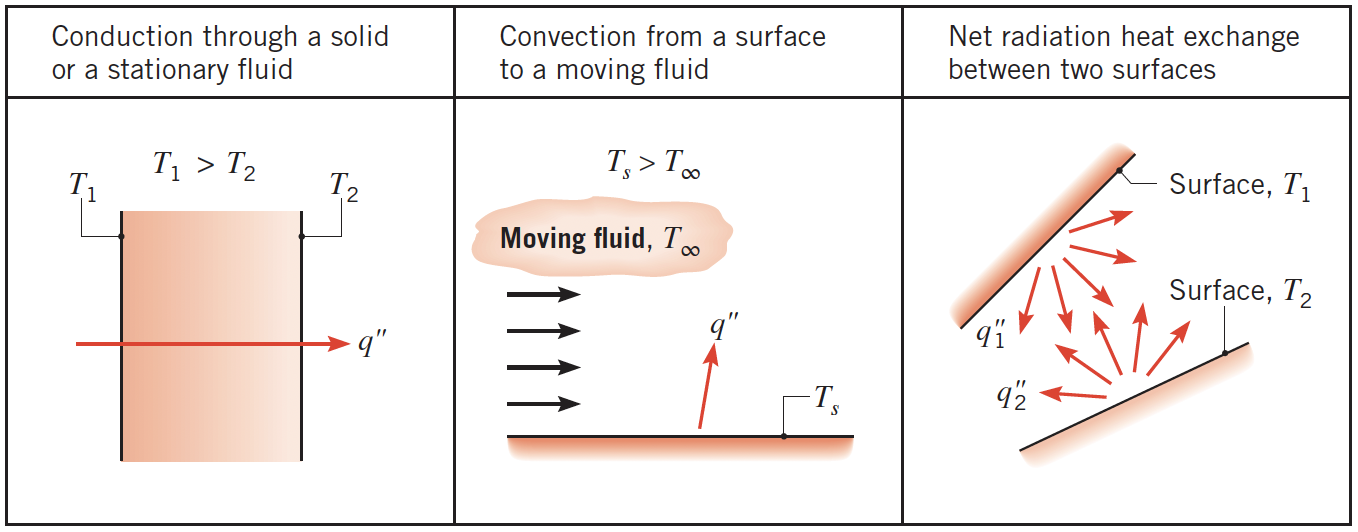
- Conduction: Energy transfer through a solid or stationary fluid via molecular interaction. This occurs from high to low temperature due to microscopic collisions and vibrations.
- Convection: Transfer between a solid surface and a moving fluid. Here, energy is transported both by molecular motion and the bulk movement of the fluid.
- Radiation: Transfer of energy through electromagnetic waves. Unlike conduction and convection, radiation does not require a medium and can occur in a vacuum.
These are illustrated schematically in Figure 1.1 of the textbook (Bergman, Lavine, and Incropera, 2019, 3), reproduced below.
Sensible vs. Latent Thermal Energy
Thermal energy itself includes:
- Sensible energy: Associated with temperature change.
- Latent energy: Associated with phase change at constant temperature (e.g., melting, vaporization) (Bergman, Lavine, and Incropera, 2019, 16).
Both are relevant in heat transfer analysis, depending on whether temperature change, phase change, or both are occurring.
Temperature Scales
Temperature can be expressed in several units depending on the application and region. In engineering contexts, we most often use:
- Kelvin (K): The SI unit of absolute temperature. Zero kelvin represents absolute zero—the point at which all thermal motion ceases.
- Celsius (°C): A relative scale based on the freezing (0°C) and boiling points (100°C) of water at 1 atm.
- Fahrenheit (°F): Commonly used in the United States. The freezing point of water is 32°F and the boiling point is 212°F.
- Rankine (°R): An absolute temperature scale based on fahrenheit units, used primarily in thermodynamic calculations in some U.S. engineering contexts.
Conversions among these scales are straightforward:
\[ T(°\mathrm{F}) = 32 + \frac{9}{5} T(°\mathrm{C}) \]
\[ T(\mathrm{K}) = T(°\mathrm{C}) + 273.15 \]
\[ T(°\mathrm{R}) = T(°\mathrm{F}) + 459.67 = \frac{9}{5} T(\mathrm{K}) \]
Note that celsius and fahrenheit are relative temperature scales (with arbitrary zeros), whereas kelvin and rankine are absolute scales because they are numerically \(0\) at absolute zero, the point at which all molecular motion ceases.
Bibliography
- [BLI] Bergman, T. L., Adrienne Lavine, and Frank P. Incropera. Fundamentals of Heat and Mass Transfer. John Wiley & Sons, Inc..